This activity uses a push/pull balance, equal-volume masses, a displacement vessel, and a graduated cylinder to explore mass and density. The activity take 40-45 minutes. Intended for ages 11 and up.
What you'll need:
- Push/pull Balance 500 g (PH0035B)
- Lead-Free Density Block Set with Hooks – 32mm Cubes (PH0108PCBLF)
- Displacement vessel (overflow can) 250 mL (PH0119C)
- Graduated Cylinder 50 mL (CH0354C)
- Pencil and paper to record your observations
- Paperclip to carefully lower the masses into the displacement vessel




Activity Guide:
- Calibrate the push/pull balance. Hold the balance by the long c-shaped handle and look at the scale on the side. Rotate the white screw so that bottom of the angled section lines up with the top-line of the scale. In the images below, the first is too high, the second is too low, and the third is just right.



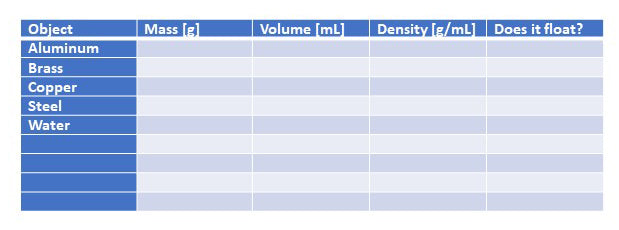
- Measure the weight of the 4 cubes using the push/pull scale. The mass is given on the right side of the scale and is measured in grams. Record your observations in the chart below.
- At your kitchen sink, fill the displacement vessel with water to the top so that water spills out the nozzle into the sink.
- After the water stops flowing out of the displacement vessel, hold the graduated cylinder below the nozzle and then lower a cube into the displacement vessel. You need to catch all of the water from the displacement vessel.
- Read the volume of water that you caught in the graduated cylinder. This gives the volume of the cube which displaced the water. Record your result.
- Repeat steps 3-5 for all of the cubes. Be sure to do step 3 for each cube to get accurate results.
- Calculate the density, p, of the cubes by dividing the mass, m, by the volume, V, as shown in the following equation.
p = m/V
Further Exploration:
- Measure the density of other objects around your house. If the object floats, you will need to devise a method for holding it under the water to get the volume measurement. Perhaps you can use paperclips to do this. Whatever your method, be sure the volume of the method for submerging the object doesn’t affect your volume measurement. For example, holding down an object with your finger would displace much more water than the actual object.
- Devise a method for measuring the density of water.
- Devise a method for measuring the density of ice.
- How can you determine if an object will float if all you know if its density
- Devise a method for measuring the density of vegetable oil. Can you find or construct an object which will float in water but sink in oil? Hint – try attaching something denser than water to an object less dense than oil, like silly putty and wood. Too much putty and it will sink. Not enough putty and it will float. So somewhere in between should allow it to float on water but sink in oil.
Data Table: